Autoimmune disease market landscape
AIM Bios are a platform technology with potential to revolutionize treatment options in numerous multi-billion $ disease markets (MS, PD, T1D, RA, IBD, …). MOGAD and NMOSD are orphan diseases (prevalence 1.3–2.5 100,000) with high medical need, and in the case of MOGAD no approved therapeutics. This should facilitate clinical entry and accelerate approval. Extension of use of the MOGAD compound for MS appears feasible. MS, T1D and PD are common autoimmune diseases with a high medical need (~10 years lower life expectancy in T1D). Current therapeutics strongly impair the daily lives of patients. AIM Bios could stop disease progression and extend intervals between treatments from several injections per day to one injection every 1-2 months.
Partnering opportunities
IND enabling / early clinical development partnerships / investments
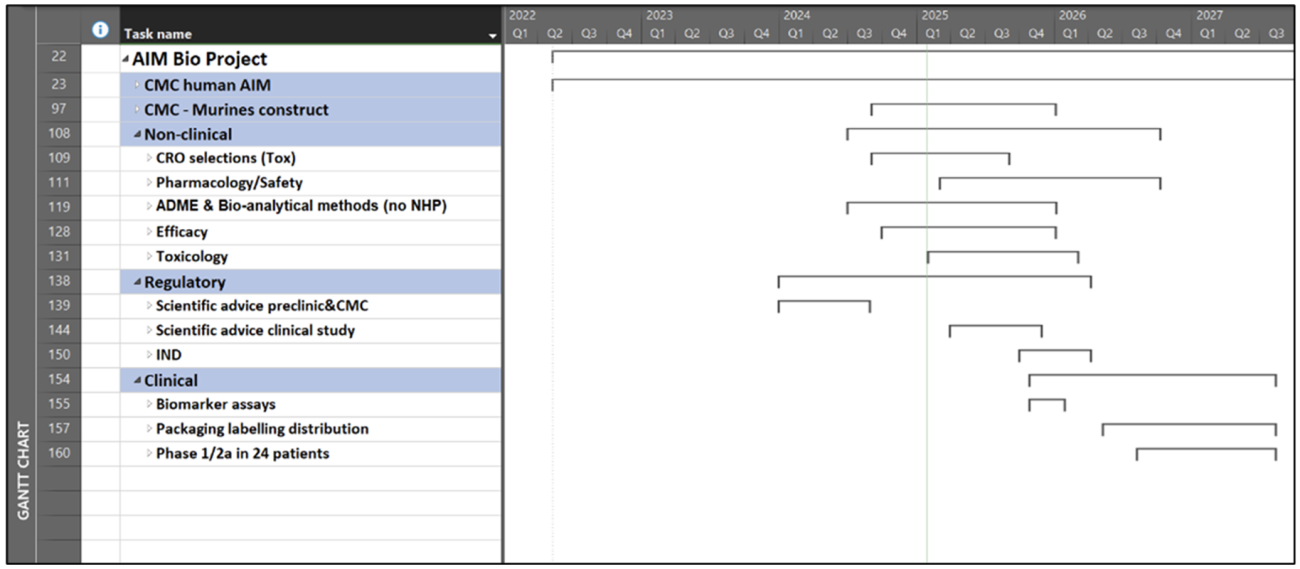
A lean IND-filing program for TOL101 was supported by the German regulatory authority (PEI) for AIM Bio lead program TOL101 (MOGAD) in September 2024:
- Preclinical proof of principal in suitable in vivo and in vitro in systems already provided
- One binding assay sufficient for release and stability testing
- PK/TOX with mouse-adapted AIM Bio in mice sufficient
- Maximum recommended starting dose (MRSD) to be derived from in vitro and in vivo experiments
- Quality development largely comparable to other biologics (antibodies)
Similar regulatory developments should apply to our other AIM Bio programs such as NMOSD or Parkinson‘s disease.
Toleris is looking for biotech or pharmaceutical companies interested in co-developing or venture capital partners interested in investing in our company or selective more advanced therapeutic programs. These are MS and MOGAD, for which we have received regulatory scientific advice, and type 1 diabetes, for which we have prioritized human candidates.
Preclinical development partnerships / early investments
Toleris is also looking for very early investors, biotech and academic partners to adapt the AIM Bio platform to novel indications. We offer extensive experience in human candidate or surrogate AIM Bio design, production and prioritization, and have successfully collaborated with academic and clinical partners in Europe and the US. Opportunities include myasthenia gravis, pemphigus vulgaris, stiff person syndrome, rheumatoid arthritis and many other autoimmune diseases with defined autoantigens.
Please contact us to receive further information or set up a video call!