The AIM Bio platform for targeted immunological tolerance induction
Summary
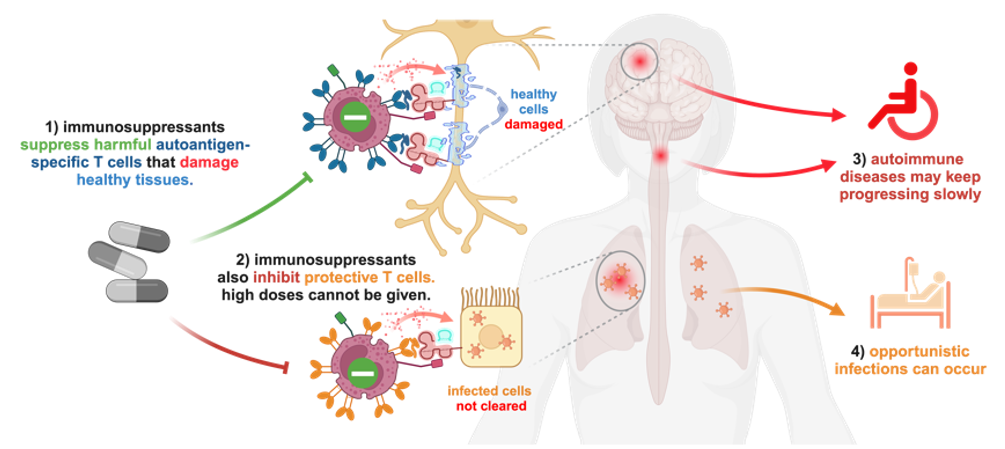
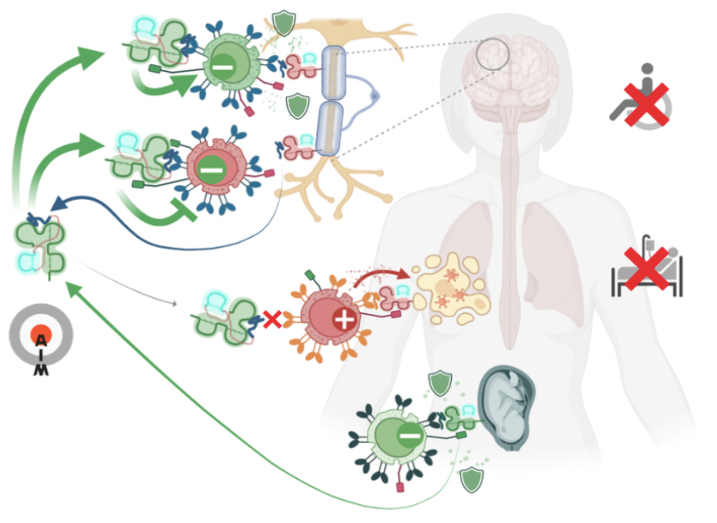
Autoimmunity Modifying Biologicals (AIM Biologicals) are soluble, near-physiological proteins capable of inducing selective, antigen-specific immunological tolerance in an organ of choice. They mimic a natural principle to protect embryos from the maternal immune system. Autoimmune diseases are driven by rare autoantigen-specific T cells that recognize autoantigens presented on classical MHC molecules. This leads to direct attacks on healthy organs, secretion of pro-inflammatory cytokines or activation of other immune cells such as autoantibody-producing B cells. Immunosuppressants inhibit both autoreactive T cells and protective immune functions (Fig.1 1). Thus, doses required to fully stop progression of the autoimmune disease (Fig.1 3) would greatly increase the risk for severe opportunistic infections (Fig.1 4).
Figure 1: Potential and limitations of broadly acting immunosuppressants in autoimmunity
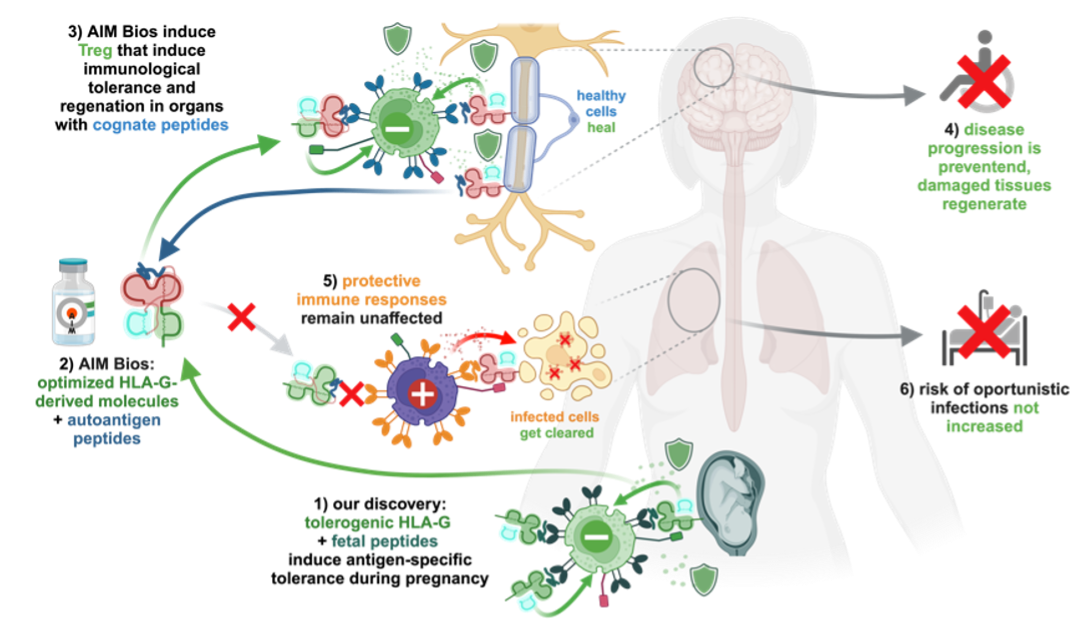
We discovered that soluble variants of the non-classical MHC Ib molecule HLA-G accumulate in lymph nodes where they induce selective immune tolerance towards the presented peptides. Induced regulatory CD8+ T cells can then protect tissues presenting their cognate antigen. Physiologically, this enables embryos to specifically tolerize the maternal immune system for fetal antigens expressed in placenta (Fig.2 1). AIM Biologicals use optimized HLA-G to induce similarly targeted and effective tolerance towards autoantigens attacked during autoimmune diseases (Fig.2 2). Successful tolerance induction against one tissue antigen also confers bystander protection when further autoaggressive T cells attack the tissue (Fig.2 3). Systemically, these therapeutics should not affect beneficial immune responses against pathogens (Fig.2 5). Thus, AIM Bios are expected to stop autoimmune disease progression (Fig.2 4) without promoting oppor-tunistic infections (Fig.2 6).
Figure 2: AIM Bios: targeted immune tolerance inspired by pregnancy
Introduction
Autoimmune diseases
There are over 80 recognized autoimmune diseases. Approximately 10% of the world population are affected by these conditions. Common examples include type 1 diabetes (T1D), multiple sclerosis (MS), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and autoimmune thyroid diseases. Autoimmune diseases are notable for their growing incidence and prevalence. They have significant economic and health impacts, contributing to morbidity and disability.
Treating autoimmune diseases poses substantial challenges, as current therapies often rely on generalized immunosuppression. While effective in reducing inflammation and immune-mediated damage, these treatments impair the body’s ability to fight infections. There is an unmet medical need for precision immunosuppressants, which selectively target pathogenic immune responses while preserving overall immune function. Successful therapeutic induction of antigen-specific tolerance thus has the potential to fundamentally change the treatment of autoimmune diseases.
Embryo-specific immune tolerance: learning from evolution
Most organ transplants require meticulous MHC matching and strong immunosuppressants to avoid rejection. Embryos expressing paternal MHC and antigens are, in contrast, selectively tolerated by the maternal immune system. This was attributed to the placenta acting as a physical barrier. However, we now know that fetal cells also circulate in maternal blood, where they can be isolated for prenatal genetic testing. Surprisingly, these cells are also tolerated by the maternal immune system. Studies in mice have even shown that paternal skin transplants are tolerated during pregnancy, while unrelated grafts are rejected.
Tolerance-inducing mechanisms developed under maximum evolutionary pressure to enable pregnancy. Harnessing a natural mechanism via near-physiological biotherapeutics offers several distinct advantages:
- High Specificity: Selective tolerance induction as in pregnancy can result in a precise targeting of unwanted immune responses, minimizing side effects and eventual toxicity.
- High Efficacy: By leveraging potent biological mechanisms, which are hard-wired in the human immune system, these therapies achieve significant therapeutic impact.
- Human Compatibility: The risk of immunogenicity and adverse immune reactions are expected to be low for almost or completely human-identical biologicals.
Broad applicability: Feto-maternal immune tolerance requires tolerization towards a multitude of paternal antigens (including often three major MHC mismatches). Tolerizing biomolecules thus have to be flexible enough to direct tolerance towards specific target proteins or cells. This could best be achieved by combining a specificity- with a tolerance-inducing signal. Such a combination would be broadly applicable for therapy.
The non-classical MHC Class Ib Molecule HLA-G
Among the numerous immunoregulatory molecules involved in immune tolerance during pregnancy, MHC class Ib molecules are of particular interest. While known to be potently immunosuppressive, they also present peptide antigens (PMID: 28904123). This antigen-presenting function would allow selective tolerance induction towards embryo-derived peptides without inhibiting immune responses to pathogens.
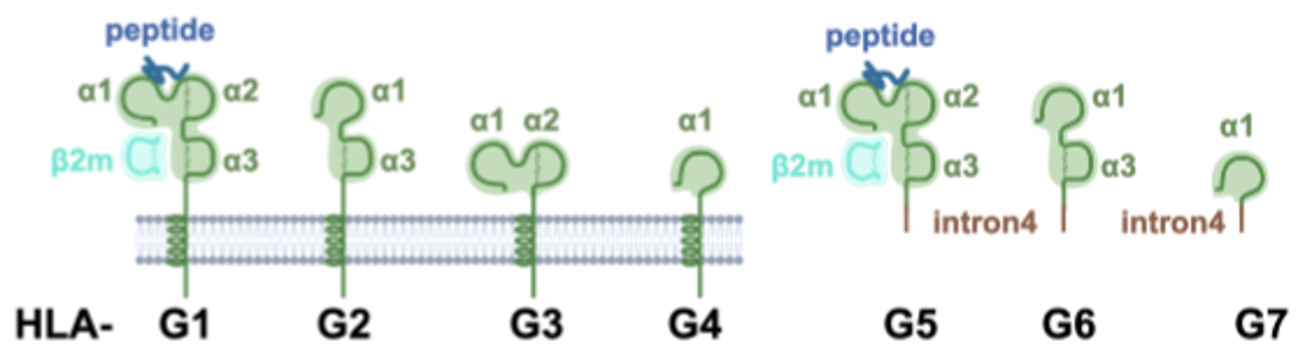
HLA-G is the main pregnancy-associated non-classical major histocompatibility complex (MHC) class I molecule. Unlike classical MHC molecules, it shows only very limited polymorphism and is primarily involved in immune tolerance (PMID: 18249584). HLA-G is expressed in both membrane-bound and soluble forms. Toleris scientists have shown that soluble HLA-G can stick to the surface of antigen-presenting cells (APCs), where it remains functional. It is highly expressed in the placenta, where it plays a key role in protecting the fetus from maternal immune rejection (PMID: 18249584).
Figure 3: membrane-bound (HLA-G1-G4) and soluble (HLA-G5-G7) isoforms of HLA-G
In transplantation, HLA-G promotes graft acceptance and reduces rejection due to its immunosuppressive properties (PMID: 18249584). Endogenous HLA-G expression in transplanted organs correlates with improved outcome and tolerance induction (PMID: 35769475). In autoimmune diseases, altered HLA-G expression may reflect its role in modulating aberrant immune responses. Elevated HLA-G levels in systemic lupus erythematosus patients are thought to contribute to immune homeostasis (PMID: 21395561). Just like classical HLAs, HLA-G is known to present peptide antigens (PMID: 8805247). Nevertheless, the role of the presented peptides had hardly been explored (PMID: 34948145).
HLA-G interacts with several immune-modulatory receptors
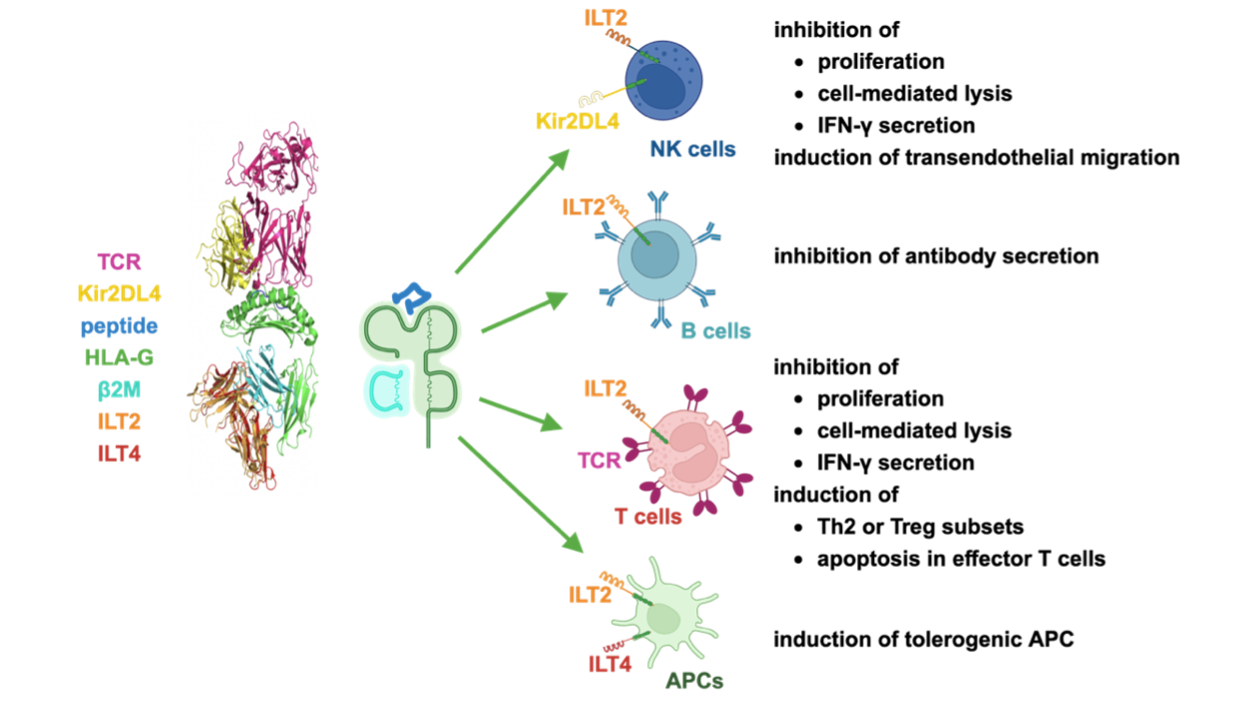
HLA-G inhibits immune responses by interacting with receptors like ILT2, ILT4, and KIR2DL4, suppressing natural killer (NK) and T-cell activity (PMID: 8829012). We found that soluble HLA-G molecules bind to the surface of antigen-presenting cells (APCs), primarily via ILT4/LILRB2. When attached to the surface of APCs, these soluble HLA-G molecules can still interact with T cells. This mechanism enables embryo- or tumor-derived HLA-G molecules to present embryo- or tumor-derived antigens in draining lymph nodes where they can potently influence the priming of host immune responses. These considerations prompted us to explore whether HLA-G-related soluble molecules can induce antigen-specific immune tolerance.
Figure 4: HLA-G inhibits various immune cells mostly via ILT2, ILT4 and KIR2DL4 receptors.
Design and AIM Bio generations
To explore antigen-specific effects of HLA-G, we generated HLA-G-derived molecules that allow for studying these effects in model systems. Importantly, these could also be used as therapeutic agents.
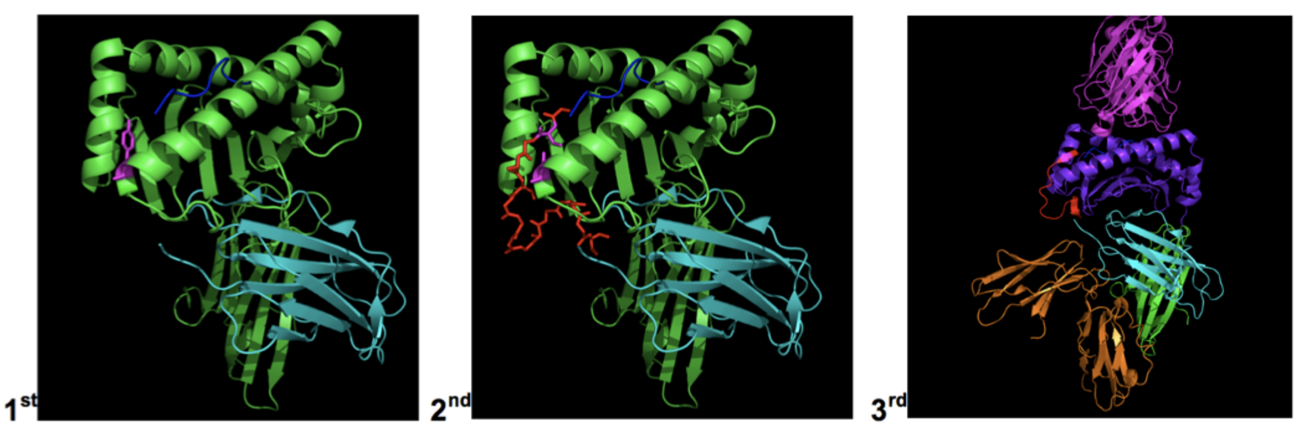
First, we designed soluble molecules comprising a peptide antigen, HLA-G and b2-microglobulin (b2m) and connected these three molecules via covalent linkers (2nd generation AIM Biologicals). In 3rd generation AIM Biologicals, we also exchanged the HLA-G a1 and a2 domains for antigen-binding a1 and a2 domains from other MHC class I molecules (Figure 5). This enabled the presentation of well-characterized human or murine antigens in the presence of the tolerogenic HLA-G α3 domain.
Figure 5: AIM Bio candidate generations. In 1st generation AIM Bios, the presented peptide was non-covalently linked to HLA-G. In 2nd generation AIM Biologicals, linkers were introduced to covalently couple the peptide to β2m and β2m to HLA-G. In 3rd generation AIM Biologicals, alternative peptide-presenting α1 and α2 domains were introduced. Receptors ILT2 and the T cell receptor (TCR) are also shown.
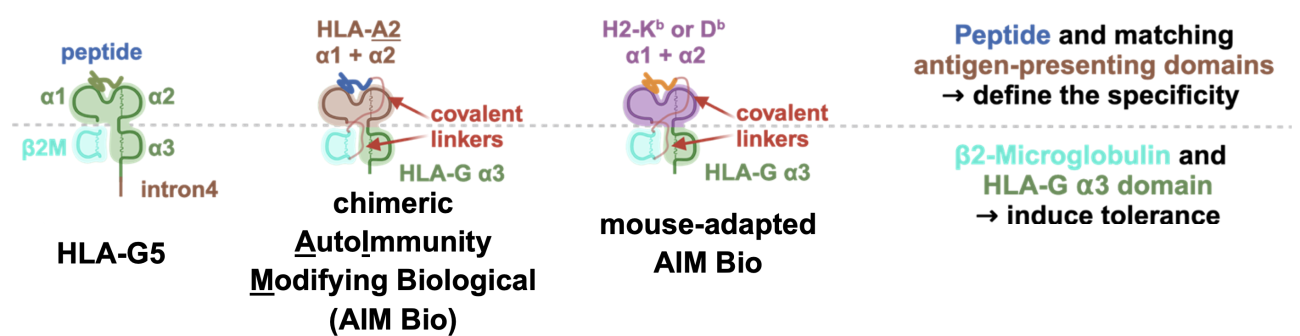
Using chimeric HLA-G derived molecules greatly enhances the flexibility and versatility of the AIM Bios platform. As the initial frequency of T cells recognizing a specific antigen is very low (often below 1 in 100,000), we first designed AIM Biologicals specific for human model antigens or for murine MHC molecules (for use in animal studies). We thus modified the natural soluble human HLA-G5 molecule to allow presentation of antigens recognized by comparatively frequent human HLA-A2- restricted T cells or by murine T cells from transgenic mice, in which every T cell expresses a cognate T cell receptor (TCR).
Figure 6: Design of human and mouse-adapted AIM Bios and functions of antigen-presenting and tolerance-inducing domains.
Mode of action
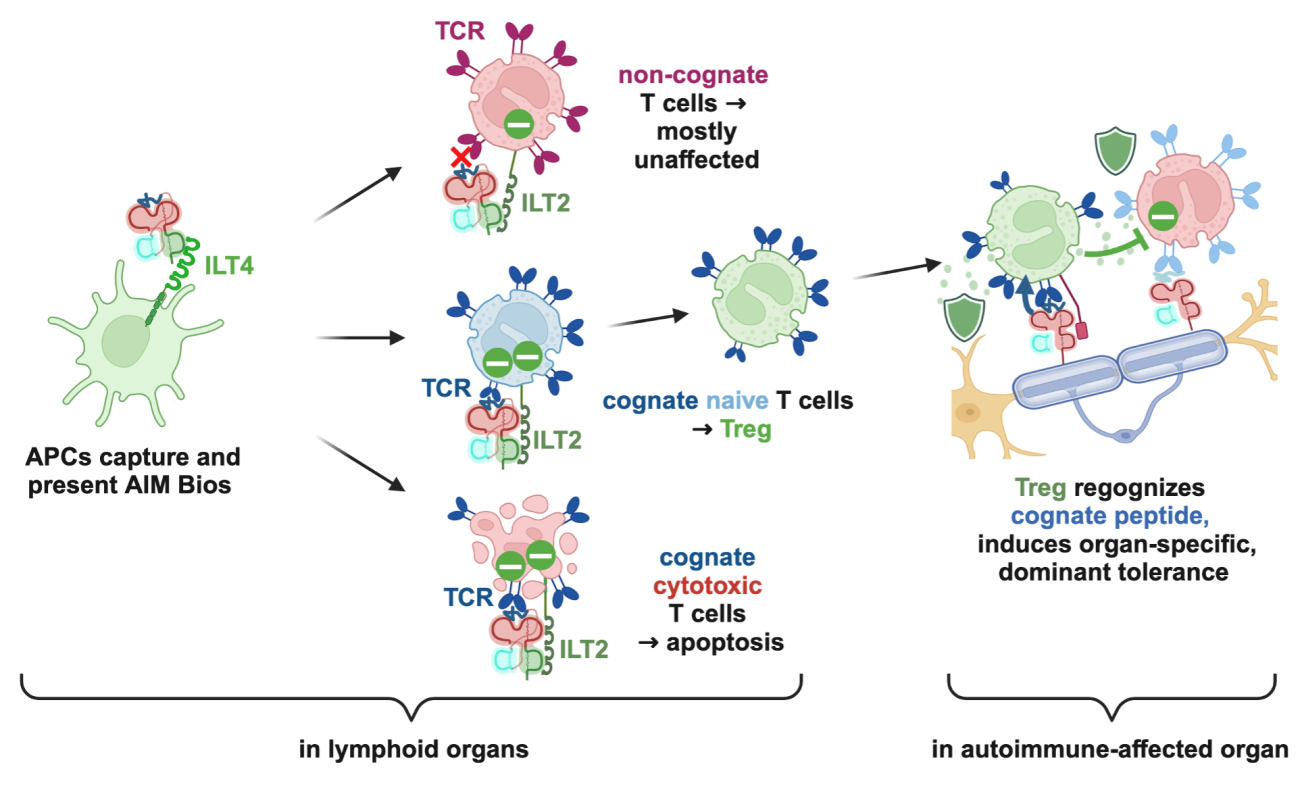
The following mode of action for AIM Bios has been experimentally confirmed in vitro and in mouse models in individual steps and is anticipated to also occur in patients. First, injected AIM Bios are efficiently captured by antigen-presenting cells (APCs) in the lymph nodes through interaction with the immunoglobulin-like transcript 4 (ILT4) receptor.
Localization on APCs in lymph nodes facilitates the modulation of immune responses in a highly selective manner. When these APCs interact with non-cognate effector T cells, which do not recognize the specific antigen presented by the AIM Bios on the APCs, T cells remain largely unaffected. In contrast, cognate effector T cells that are highly activated and recognize their specific antigen will likely undergo apoptosis, as shown in Fig. 8, leading to a targeted reduction in autoreactive or excessive immune responses. Additionally, naïve T cells that recognize the antigen presented by the AIM Bio, are directed towards differentiation into regulatory T cells (Treg) with a tolerogenic phenotype. These Treg then migrate to tissues affected by autoimmune conditions, where they encounter their specific peptide antigen again. This will trigger potent local immunosuppressive effects, which promote immune tolerance and tissue regeneration. Of note, in case of a viral infection, infected cells may express more than 80% viral proteins, which will not activate these Treg that would hinder the immune system from clearing the infection.
Figure 7: Anticipated mode of action of AIM Bios
Proof of Concept
AIM Bios eliminate cognate effector T cells and induce cognate Treg
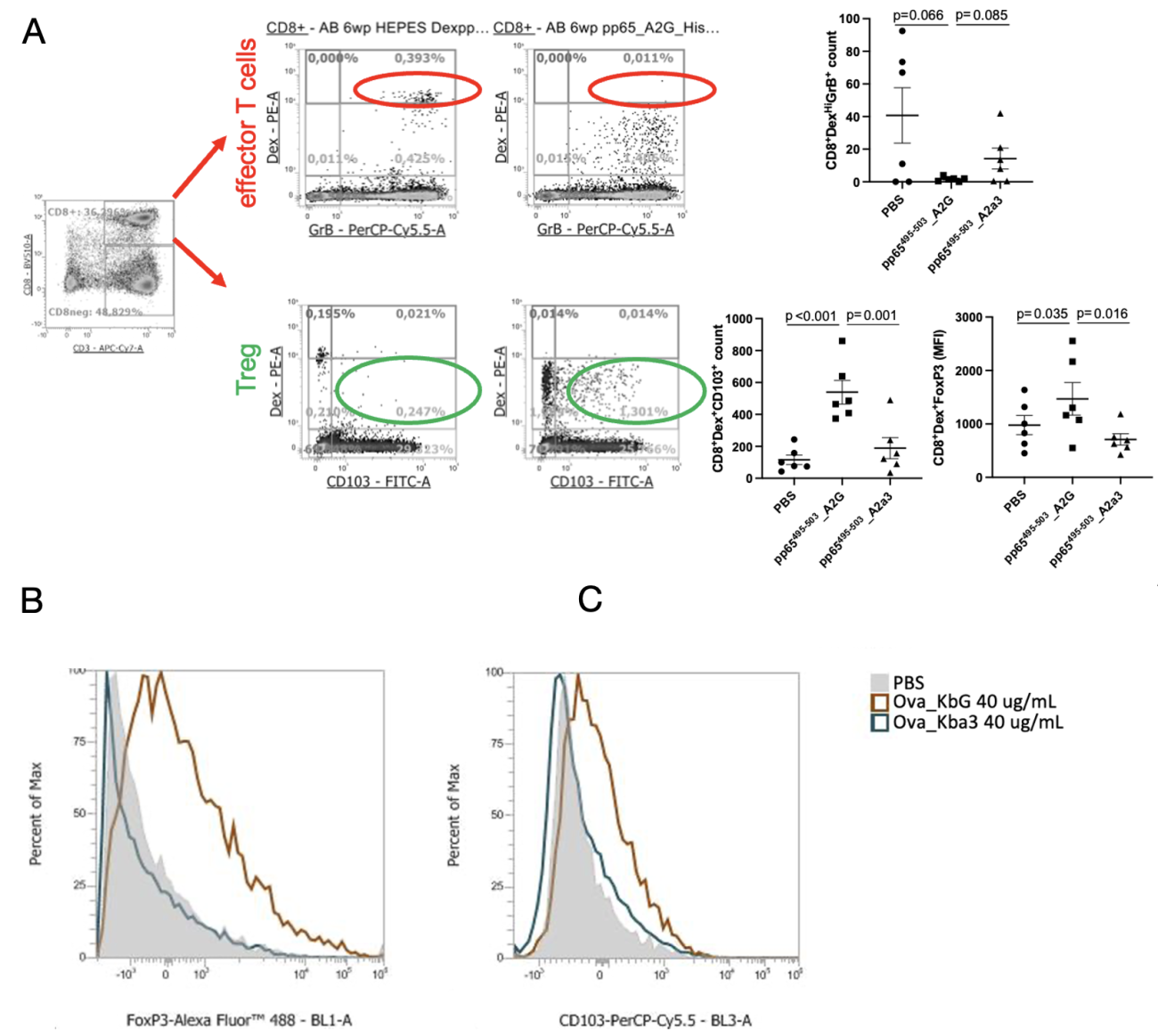
To investigate the effects of AIM Bios on human T cell responses, we used peripheral blood mononuclear cells (PBMCs) from healthy blood donors with established CD8+ T cell responses against the HLA-A2-restricted CMV PP65 antigen. We then treated these PBMCs in vitro with AIM Bios that induce tolerance to the viral peptide or with control molecules possessing an HLA-A2 instead of the tolerogenic HLA-G alpha-3 domain.
Figure 8: AIM Bios eliminate cognate effector T cells and induce cognate Treg. A) AIM Bios containing the viral peptide and the HLA-G α3 domain significantly reduced the frequency of antigen-specific Granzyme B positive effector CD8+ T cells in PBMC samples from donors with established T cell responses towards that peptide, while antigen-specific CD103+ and/or FoxP3+ Treg were induced. AIM Bios thus leverage at least two natural tolerance mechanisms to inhibit immune reactions against the presented peptide-antigen. An HLA-G-domain-dependent induction of Treg with the same markers FoxP3 (B) and CD103 (C) can also be observed in T cells from mice transgenic for the cognate TCR.
Mouse-adapted AIM Bios induce protective, antigen specific Treg
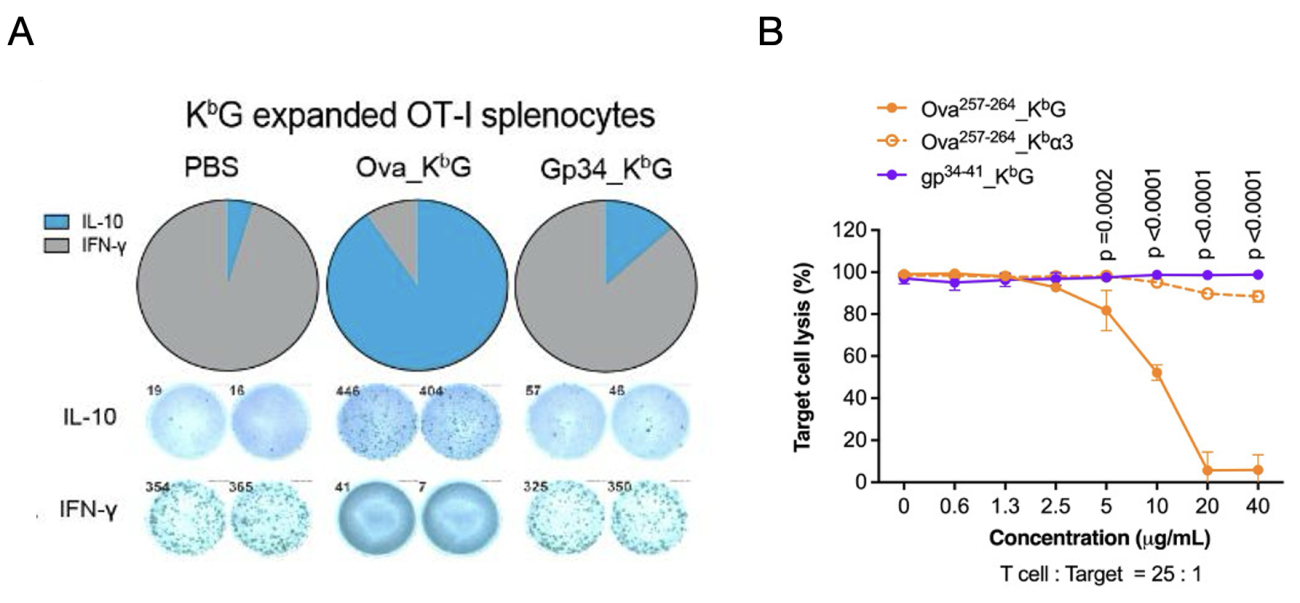
A model system to study antigen-specific effects are OT-I mice, in which all T cell recognize an ovalbumin-derived peptide epitope (Ova) presented on the MHC class I molecule H2-Kb. To study effects of AIM Bios in these mice, we generated mouse-adapted AIM Bios comprising the alpha1 and alpha2 domains of murine H2-Kb and Ova or a control peptide (viral protein gp34), combined with either the tolerogenic HLA-G alpha3 (see Fig. 6) or a non-tolerogenic H2-Kb alpha3 domain.
Within 10 days, ovalbumin-tolerance inducing AIM Bios strongly induced secretion of anti-inflammatory IL-10 (A, blue), while secretion of proinflammatory IFN-gamma (grey) was inhibited. AIM Bios presenting the control peptide had no effect. Ovalbumin-presenting AIM Bios also completely inhibit the killing of Ova-peptide-loaded target cells by OT-1 T cells in a dose-dependent manner.
Figure 9: AIM Bio surrogate molecules selectively induce IL-10 and inhibit IFN-γ secretion and target cell lysis in cognate OT-I T cells. A) Induction of tolerogenic IL-10 secreting Treg and suppression of pro-inflammatory interferon gamma was quantified by ELISpot after 48h of treatment (Ova_KbG = AIM Bio with cognate Ova peptide and H2-kb presentation domains, gp34_KbG = AIM Bio with non-cognate LCMV gp34 control peptide, mean ELISpot counts shown). B) Peptide-dependent lysis of Ova257 peptide-loaded H2-Kb positive Panc02 cells by OT-I T cells (25:1 E:T ratio) is efficiently inhibited by cognate Ova_KbG AIM Biologicals. Cognate Ova_Kbα3 molecules containing the H2-Kb (instead of the HLA-G) α3 domain do not inhibit killing.
AIM Bio-induced Treg confer antigen-dependent bystander protection
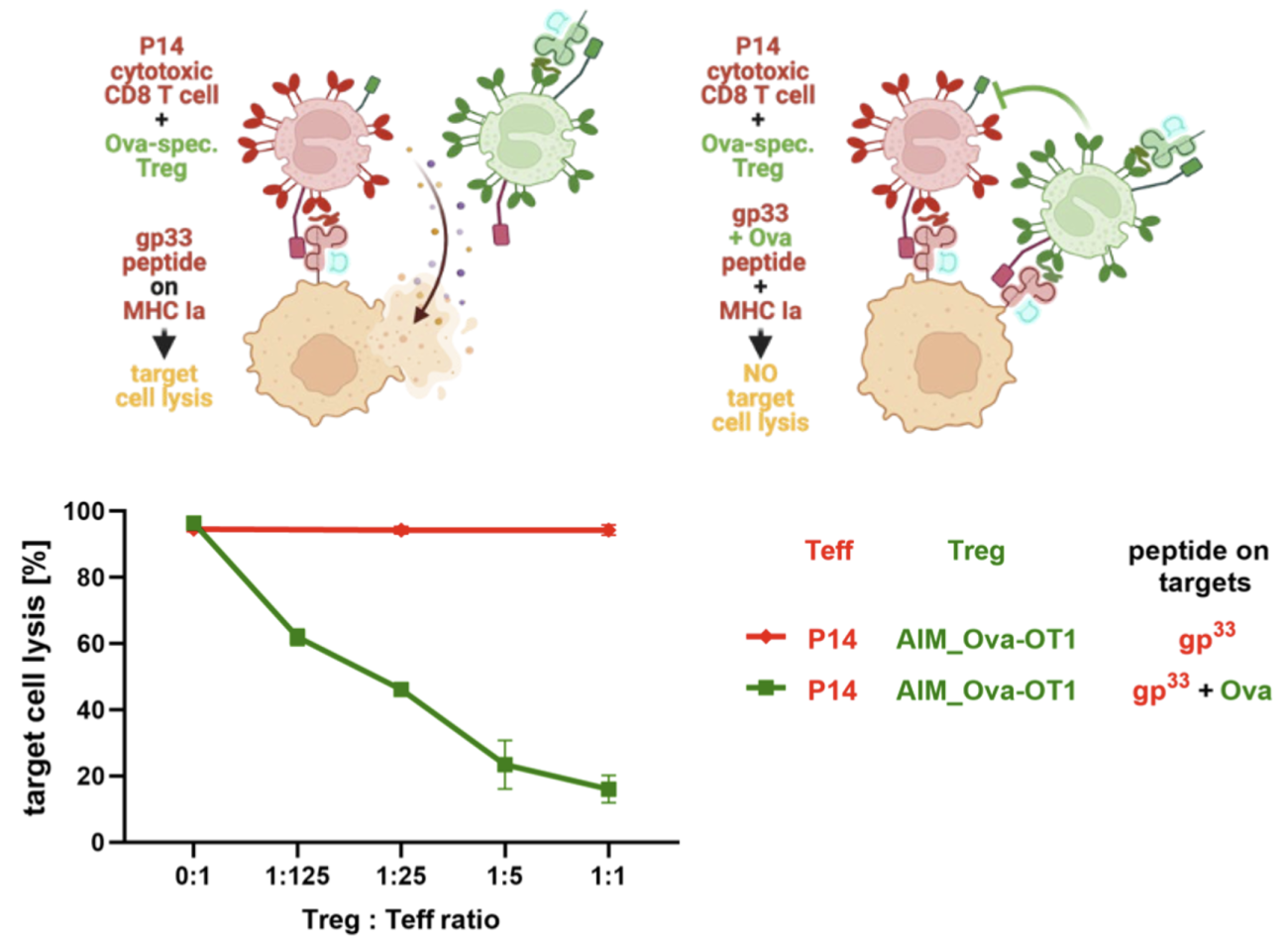
A key challenge in antigen-specific immunotherapy is that multiple antigens, many of them unknown, are targeted in most autoimmune disorders. Different antigens may be targeted in different patients. Autoimmune responses may also be directed against several epitopes. Therefore, induction of “bystander tolerance”, meaning protection against cytotoxic effector T cells (Teff) targeting a different epitope or antigen on the same cell, is considered critical for the success of antigen-specific immunotherapies. (PMID: 38086932) We thus tested if Ova-specific Treg induced by AIM Bios confer “bystander protection” against gp33-specific cytotoxic (P14) T cells. To this aim, we first treated Ova-specific OT-I T cells with either PBS as control or an Ova-tolerance-inducing AIM Bio molecule (Ova_KbG) for 14 days, with a second stimulation on day 7, to generate Ova specific CD8+ regulatory T cells. P14 effector T cells were activated in parallel with their cognate antigen gp33 for 5 days. Luciferase-transgenic PancO2 target cells, which can present OVA on H2-Kb and the gp33 peptide on H2-Kd were loaded with either the gp33 peptide only, or both antigens. gp33-specific effector T cells (Teff) were added to the peptide-loaded, luciferase-expressing PancO2 target cells at a 25:1 effector-to-target ratio. AIM Bio-treated OT-I Treg were added at varying Treg-to-Teff ratios of 0:1, 1:125, 1:25, 1:5, and 1:1. Remarkably, Ova-tolerance-inducing AIM Bios significantly inhibited gp33-mediated target cell lysis even at Treg-to-Teff ratios as low as 1:125. This protection is strictly dependent on the presence of Ova on target cells, indicating that AIM Bios induce tolerance only in organs expressing the antigen used for tolerization. OT-1 T cells incubated with Ova_Kbα3 conferred no such protection (data not shown).
Figure 10: AIM Bio-induced Treg confer antigen-dependent bystander protection
Thus, AIM Bio-induced CD8+ Treg need to recognize their cognate antigen to become active, which is only presented in the target tissue. In pregnancy, this is enabled by simultaneous expression of soluble and membrane-bound HLA-G. While soluble antigen-loaded HLA-G induces HLA-G-restricted CD8+ Treg in draining lymph nodes, membrane-bound antigen-loaded HLA-G ensures activation of Treg in the target tissue. An obvious advantage of CD8+ Treg, which recognize their cognate antigen on an MHC class I molecule, is the ubiquitous expression of MHC-I on nucleated cells. The better known CD4+ Treg, in contrast, recognize only antigens presented on MHC class II, which is rare to absent in most target tissues. By using chimeric HLA-G derived molecules with α1/2 domains from classical MHC, antigen recognition in the target tissue does not require HLA-G expression. Patient stratification according to the presence or absence of the required common HLA haplotypes can be done by standard HLA typing.
Induction of antigen-specific Treg in vivo
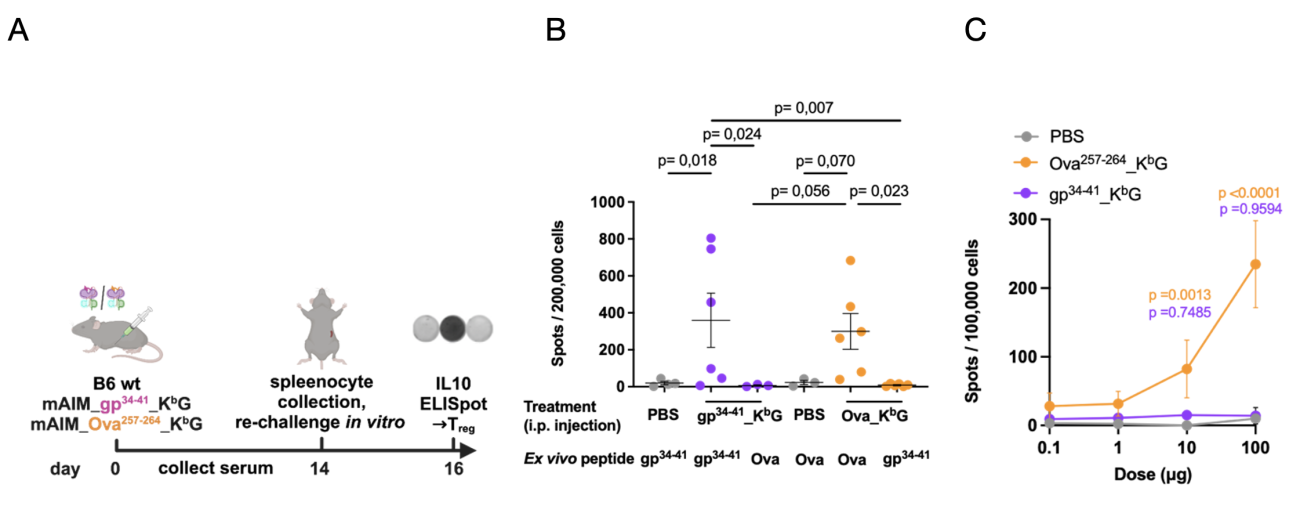
To evaluate in vivo effects of AIM Bios, wild type B6 mice were treated intraperitoneally (i.p.) with 100 μg of either Ova or gp34 tolerance-inducing AIM Bios, or PBS as a control (A). On day 14, splenocytes were harvested and re-challenged ex vivo with either peptides matching those presented on the AIM Bios or mismatched peptides. IL-10 secretion was quantified on day 16 using an ELISpot assay. A significant increase in antigen-specific regulatory cells was observed when the re-challenge peptide matched the peptide presented on the AIM Bios (B). Importantly, this experiment also shows that the gp34 tolerance-inducing AIM Bio, which was used as off-target (negative) control in subsequent experiments, is fully capable of inducing IL-10 secreting regulatory T cells.
To demonstrate dose-dependency in vivo, wild type B6 mice were treated intraperitoneally (i.p.) with 1, 10, or 100 μg of Ova or gp34 tolerance-inducing AIM Bios, or PBS as a control. After one week, splenocytes were re-challenged ex vivo with Ova peptide, and IL-10-secreting regulatory cells were quantified using an ELISpot assay. A significant, dose-dependent increase in antigen-specific regulatory cells was observed upon restimulation with Ova peptide (C).
Figure 11: AIM Bios induce peptide-dependent, antigen-specific regulatory cells in vivo
AIM Bios prevent MS symptoms and autoantibodies in mice
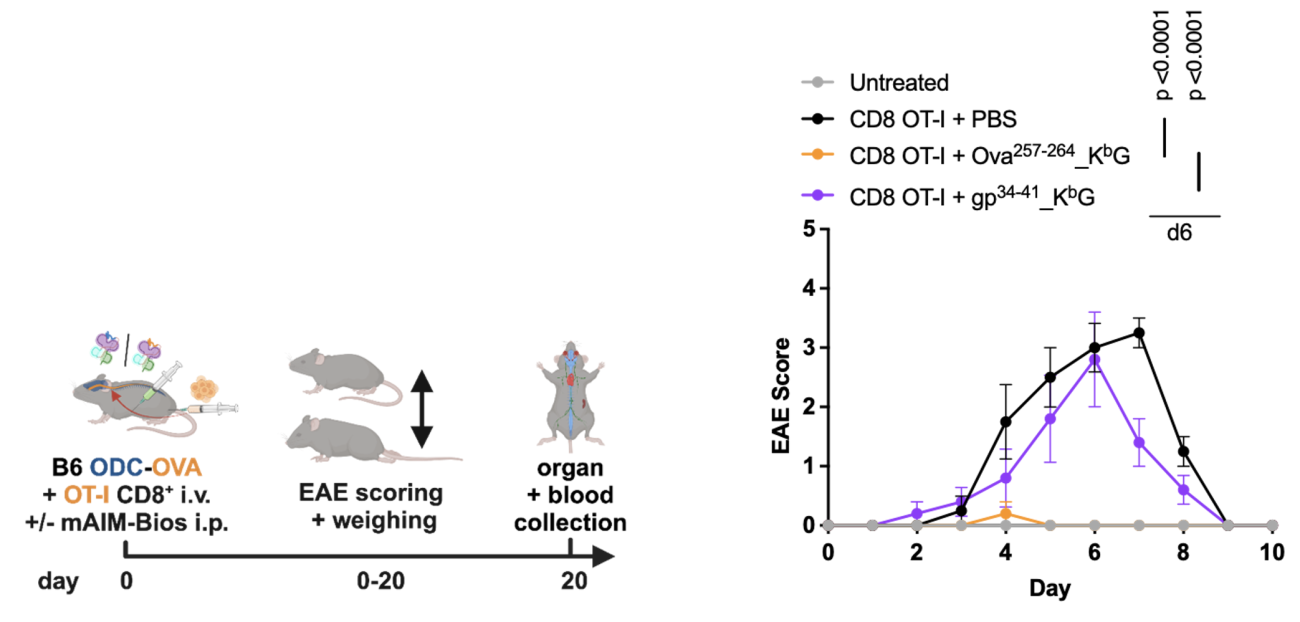
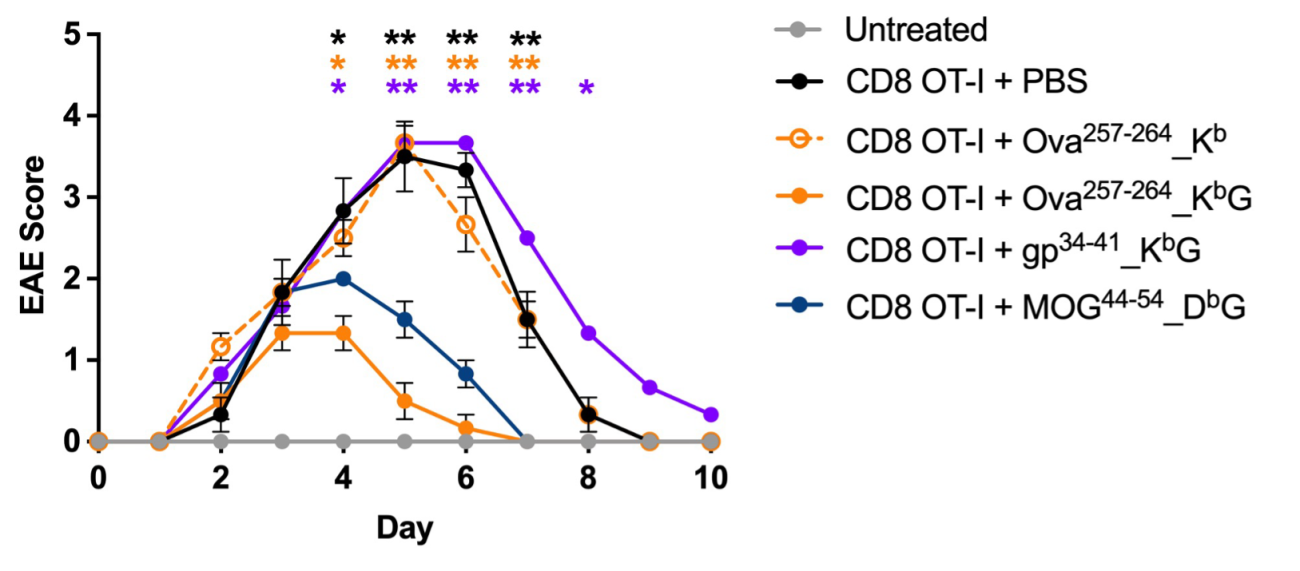
In MS patients, oligodendrocytes are attacked by myelin-reactive immune cells. In order to study if AIM Bios prevent autoimmune disease symptoms, mice expressing the Ova model antigen in oligodendrocytes were injected with Ova-specific T cells and mouse-adapted AIM Bios. Mice treated once with 500µg AIM Bios presenting the targeted Ova peptide were completely protected from paralysis (EAE), while AIM Bios presenting the gp34 control peptide had no effect.
Figure 12: AIM Bios fully prevent MS-like symptoms in the ODC-Ova EAE model
As the targeted autoantigen peptides may vary between patients, we tested if AIM Bios inducing tolerance towards the oligodendrocyte antigen MOG protect mice in which the disease is caused by Ova-specific T cells as described above. 250 µg of an AIM Bio inducing tolerance towards a MOG peptide MOG (MOG44-54_DbG) conferred almost the same level of protection as Ova-specific AIM Bios. Molecules presenting the correct Ova-peptide but lacking the HLA-G a3 domain (Ova257-264_Kb), showed no protective effect.
Figure 13: AIM Bios inducing tolerance to non-targeted peptides expressed by the same target cells also prevent disease symptoms
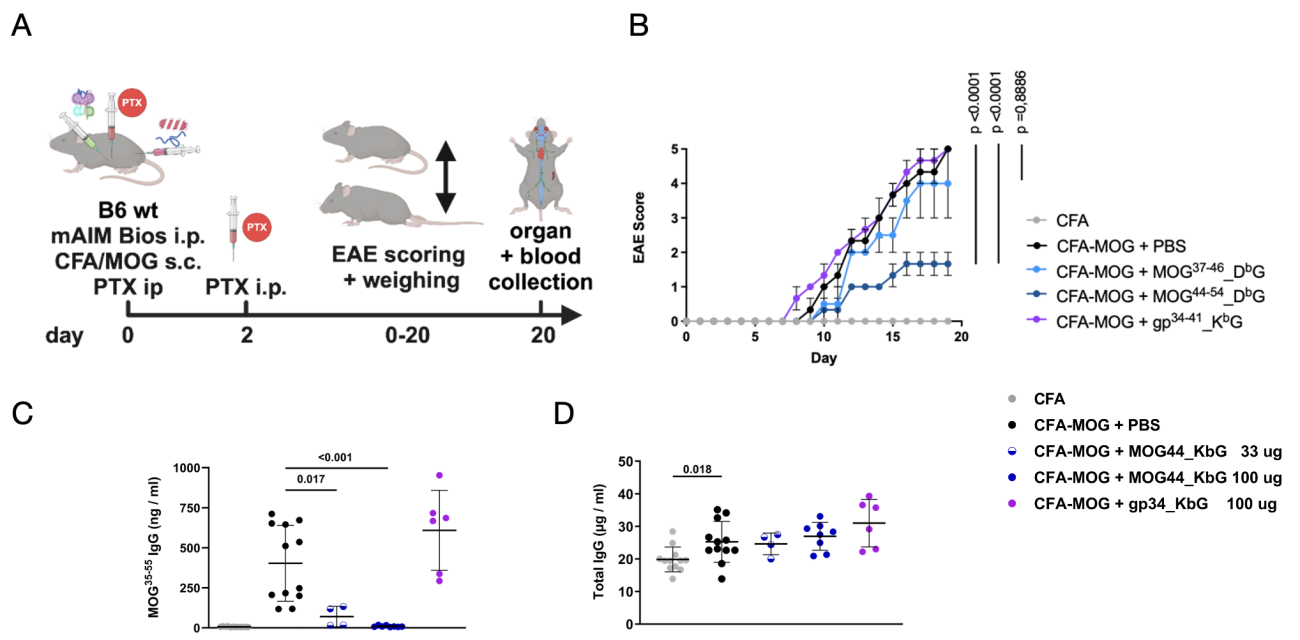
To investigate the effect of soluble MOG-specific AIM Bios on a chronic experimental autoimmune encephalomyelitis (EAE) model and on autoantibodies, MOG-tolerance inducing AIM Bios were tested in the MOG35-55 EAE mouse model for multiple sclerosis (Figure14A). 8-week-old C57BL/6J mice were immunized with either CFA-emulsified recombinant MOG35-55 peptide (to induce EAE) or CFA alone as a control. Two doses (100 µg) of pertussis toxin (PTX) were administered intraperitoneally on days 0 and 2. Control treatments included PBS (control), 100 µg of MOG44_DbG, MOG37_DbG (which was less stable, but included nevertheless) or gp34_KbG (with irrelevant control peptide). Disease progression was monitored daily for 19 days, when serum and organs were collected for further analysis. The MOG44 AIM Bio significantly reduced disease severity (Figure14B). Remarkably, the development of anti-MOG autoantibodies was completely prevented in the MOG44_DbG group (Figure14C), while the amount of total antibodies (Figure14D) was not reduced. This suggests that MOG-tolerance inducing AIM Bios effectively suppress MS symptoms, including formation of autoantibodies.
Figure 14: stable murine MOG AIM Bios prevent EAE and MOG autoantibodies
Key Properties of AIM Bios
Pharmacokinetics
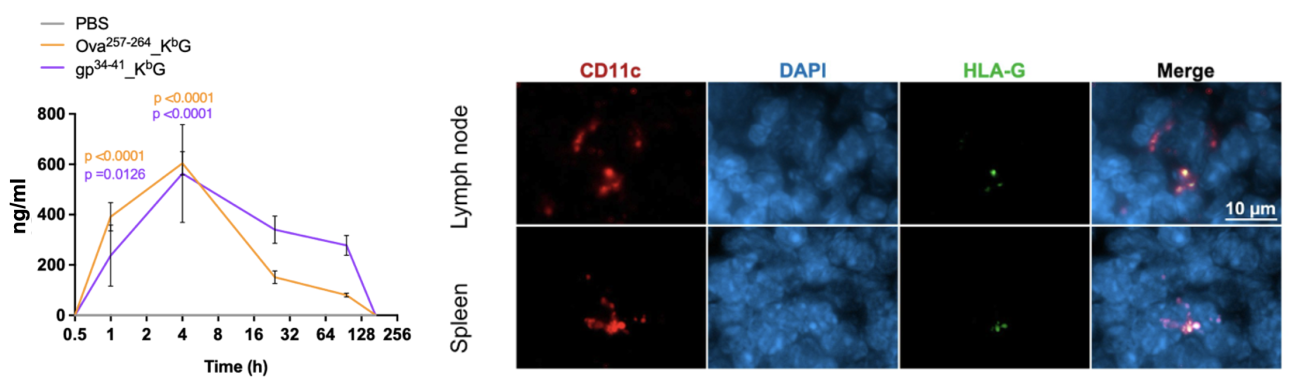
AIM Bios are 50 kDa biomolecules without Fc domain, which would typically enhance renal reuptake. Soluble HLA-G is, however, captured by antigen-presenting cells (APCs) in lymph nodes through interaction with the immunoglobulin-like transcript 4 (ILT4) receptor. As a result, intraperitoneally (i.p.) administered AIM Bios remain readily detectable on APCs in both lymph nodes and the spleen one week after injection, despite a relatively short serum half-life of approximately one day. Regarding anticipated dosing regimens in humans, it is well-established that the embryo-induced tolerogenic effects observed in autoimmune diseases during pregnancy typically fade after about three months postpartum. In our animal studies, we successfully implemented dosing schemes ranging from weekly administrations to a single dose for an entire 42-day study period. Based on these findings, we currently hypothesize that administration once per month might be a good starting point for a first clinical trial. Nevertheless, induction of Treg and tolerization of the immune response might also have permanent effects in some diseases.
Figure 16: AIM Bio serum half-life and detection of AIM Bios on CD11c+ APCs in lymph nodes and spleens 7 days after i.p. injection
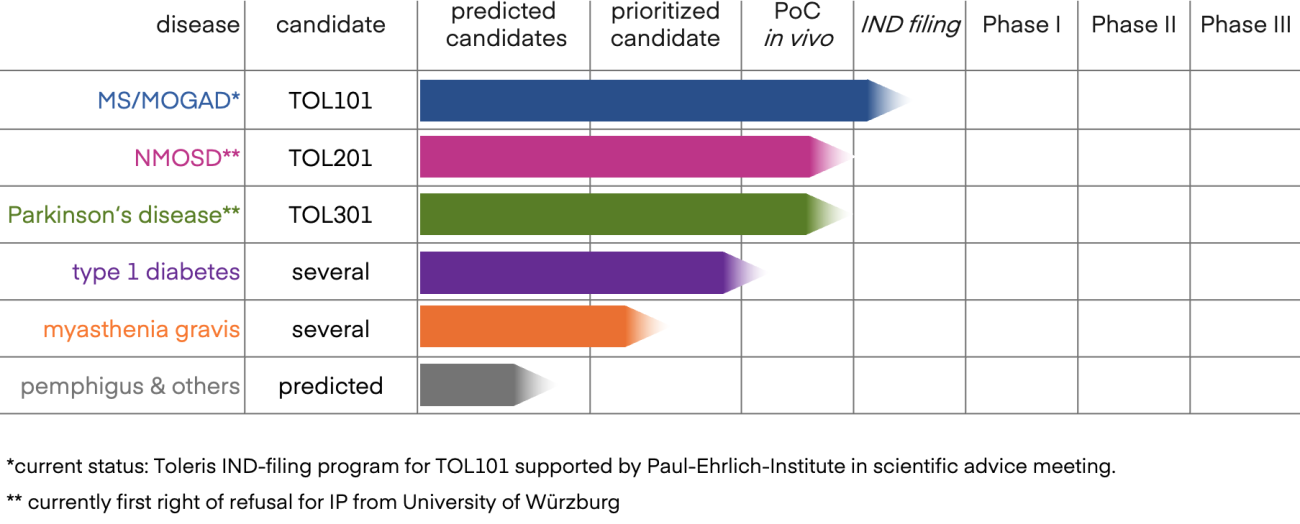
Human candidates prioritized for MOGAD/MS, NMOSD, PD and T1D
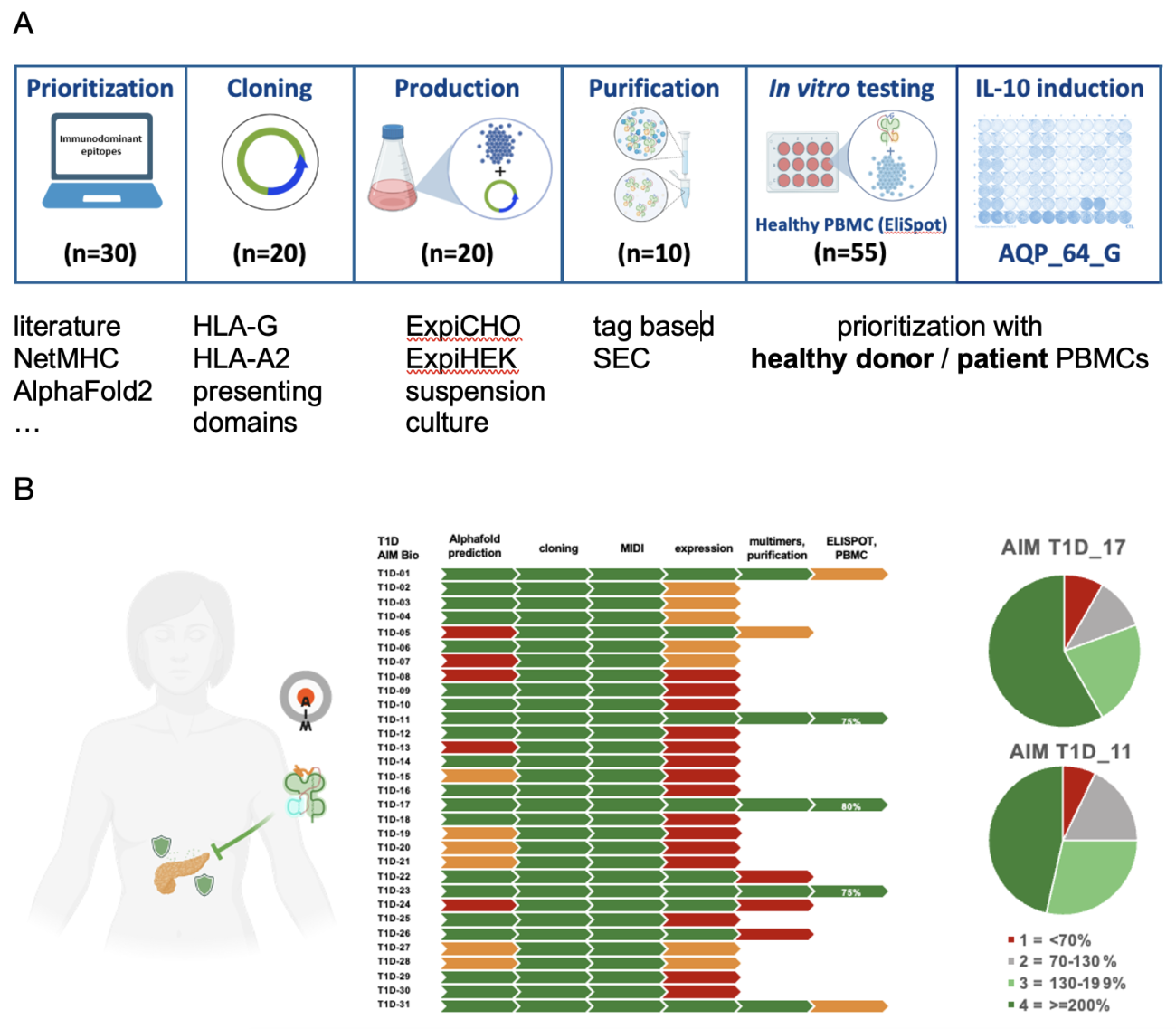
In parallel to the animal studies, we have prioritized human candidate molecules that induce regulatory T cells specific for key autoantigens for MOG antibody disease and multiple sclerosis, for NMOSD, Parkinson’s disease, and for Type I diabetes. Response to antigens presented by AIM Bios was assessed either in PBMCs from healthy donors or from patients. Separate reports on individual indications are available.
Figure 17: candidate prioritization process and results. A) >30 candidate molecules are prioritized (based on bioinformatics tools and literature analyses), cloned and expressed in suspension culture. Candidates with acceptable production yields are then tested with regard to Treg induction in (in this case 55) healthy donors and patient PBMCs. B) Overview of prioritization results for type I diabetes candidates. Good candidates induce Treg in up to 80% of healthy donor PBMCs within two weeks.
Production and characterization of AIM Biologicals
Building on internal production optimizations at Toleris, the following GMP manufacturing process is planned for AIM Bios.
Figure 18: planned AIM Bio GMP manufacturing process
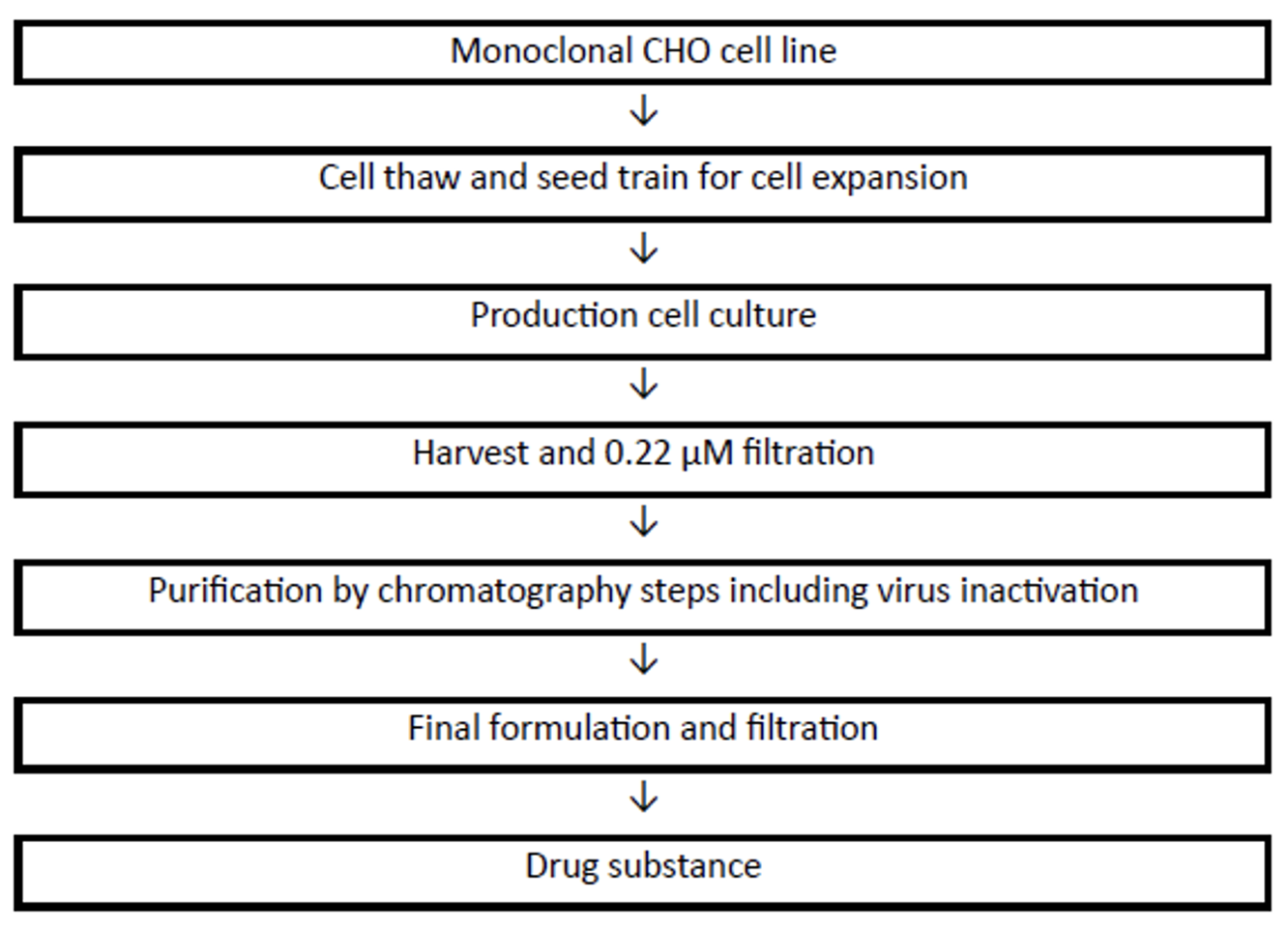
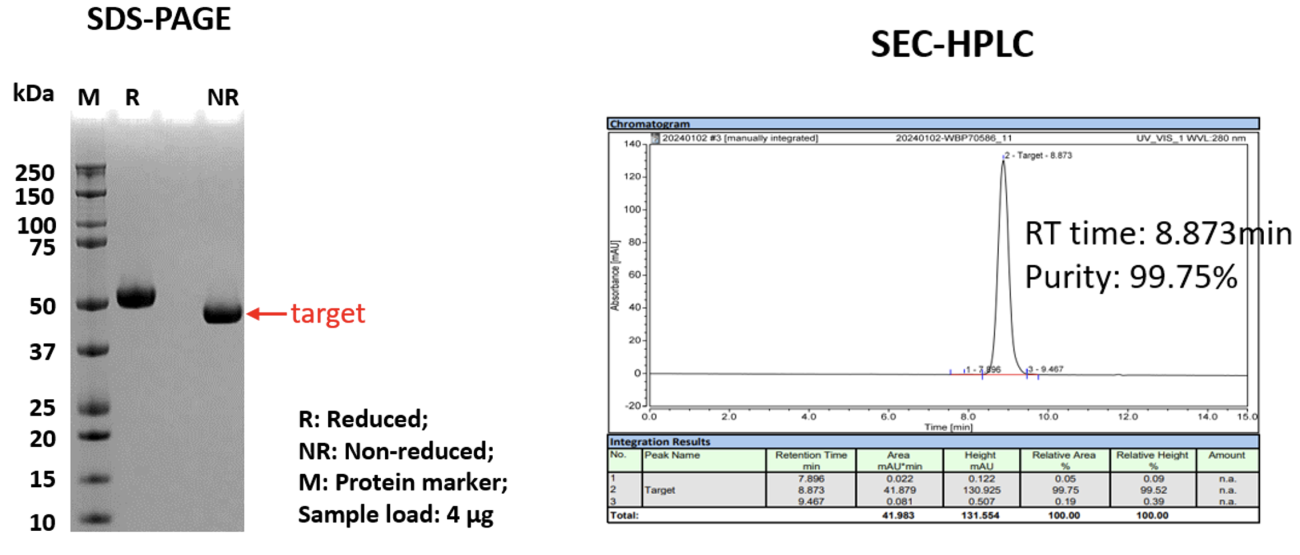
To evaluate the manufacturability of AIM Bios under GMP-like conditions, two experienced CDMOs were engaged to produce, purify, and characterize multiple AIM Bios. Internal transient expression systems yielded up to 1 mg/mL in cell culture supernatants for certain AIM Bios, while CDMOs purified >250 µg of >99% pure protein per mL of supernatant.
Figure 19: SDS-PAGE and SEC-HPLC of externally produced and purified AIM Bios
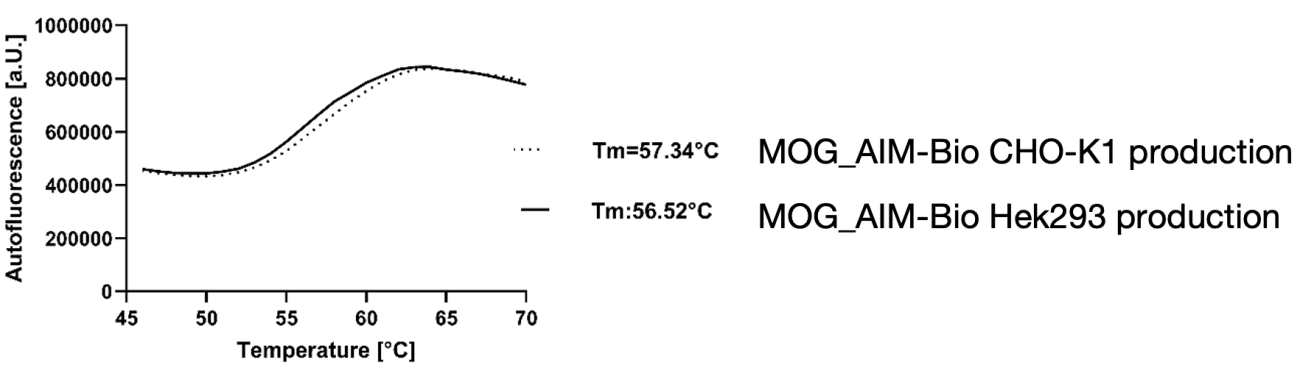
The stability of the purified AIM Bios was assessed using a Thermal Shift Assay (TSA), revealing that the melting temperatures of lead compounds—regardless of production tags—were comparable to those of monoclonal antibodies.
Figure 20: Thermal stability of AIM Bios
AIM Bios demonstrated binding to ILT2 and ILT4 in various assays, confirming their stability, functionality, and capacity to interact with immune receptors. Additionally, they were shown to induce immunomodulatory immune cells.
Unique therapeutic advantages of AIM Bios
Generally, antigen-unspecific immunosuppressive therapeutics are commonly used in autoimmune diseases to slow down disease progression. However, long-term use of such therapeutics is usually associated with severe side effects, such as opportunistic infections. Thus, there is an urgent medical need for more specific treatment options.
Antigen-specific tolerance induction has long been considered the holy grail for autoimmune treatment. Thus, it is not surprising that several approaches are under development in preclinical or early clinical development stage.
Toleris induces antigen-specific tolerance by combining an antigen with the corresponding antigen-presenting α1/2 and the HLA-G α3 domain. Competitors focus on inducing tolerance by administering antigens via liposomes, red blood cells or nanoparticles targeting the liver, immature dendritic cells or monocytes. Artificial antigen-specific cells and petide MHC nanomedicines have just entered a first clinical phase in October 2024. Administration of tolerogenic dendritic cells requires an individually tailored cell therapy product for every patient. As with CAR T cells, these cellular products are very expensive and not available off the shelf.
Toleris approach is unique in combining both the potent tolerance-inducing HLA-G domain and the specific antigen domains in an almost completely physiological molecule, which evolved over millions of years to safely and reliably induce immunological tolerance during pregnancy (references: PMID:17653127, PMID:26364507). AIM Bios are first in class biologicals inducing antigen-specific CD8+ regulatory T cells, that have been shown to be highly potent in taming autoimmune disease in mouse models. In contrast to CD4+ regulatory T cells, which can only recognize antigen presented on MHC II by professional antigen presenting cells predominantly in the lymphatic tissue, CD8+ regulatory cells can be reactivated in addition by cells within the tissue presenting the autoantigen on MHC I molecules.
Toleris AIM Bio pipeline overview
Toleris currently focuses on developing AIM Bios for neuroinflammatory autoimmune diseases such as multiple sclerosis (MS), MOG antibody disease (MOGAD), neuromyelitis optica spectrum disorders (NMOSD) and Parkinson‘s disease, as well as for type 1 diabetes.
Conclusions
Physiological de novo expression of HLA-G is likely required for pregnancy. The most frequent polymorphism in HLA-G, an insertion of 14 bp at the 3′ untranslated region (3′ UTR) in exon 8, which may affect HLA-G mRNA stability, is associated with recurrent pregnancy loss (PMID: 31280950). HLA-G molecules expressed in the placenta are loaded with fetal antigens. Loaded HLA-G molecules can selectively delete antigen-specific effector cells that could otherwise harm the embryo. Soluble, antigen-loaded HLA-G molecules will also drain to the lymph nodes. Naïve T cells recognizing fetal tissue antigens presented on HLA-G will then be polarized towards a regulatory phenotype. These (mostly) CD8+ Treg are highly antigen dependent. Therefore, they do not suppress in tissues where their cognate antigen is not presented. In placenta, however, they become dominant antigen-specific regulatory T cells.
AIM Bios leverage this newly discovered potent mechanism for targeted tolerance induction, but have been further optimized to improve manufacturability, stability, and restimulation in affected tissues. The platform has been shown to induce potent, antigen-specific tolerance in numerous autoimmune disease models. Considering that serum levels of soluble HLA-G are typically >60 ng/ml during pregnancy, excellent tolerability is expected (and was observed in all model systems). Due to their precision and simplicity, AIM Bios could thus become a true game changer for the treatment of autoimmune diseases.
Interested in learning more? Please contact us!